
De Broglie Hipotezi1 [Temel Kavramlar] YouTube
Fizicianul francez Louis de Broglie (1892-1987) a formulat, în 1923, ipoteza că orice particulă în mișcare are și proprietăți ondulatorii. 🔦 Observație Louis de Broglie a primit Premiul Nobel în 1929. Legătura dintre frecvența undei (ν) de energia particulei (E) este dată de relația:

De Broglie Hypothesis YouTube
The de Broglie‐Bohr model of the hydrogen atom presented here treats the electron as a particle on a ring with wave‐like properties. λ = h mev λ = h m e v. de Broglie's hypothesis that matter has wave-like properties. nλ = 2πr n λ = 2 π r. The consequence of de Broglieʹs hypothesis; an integral number of wavelengths must fit within.
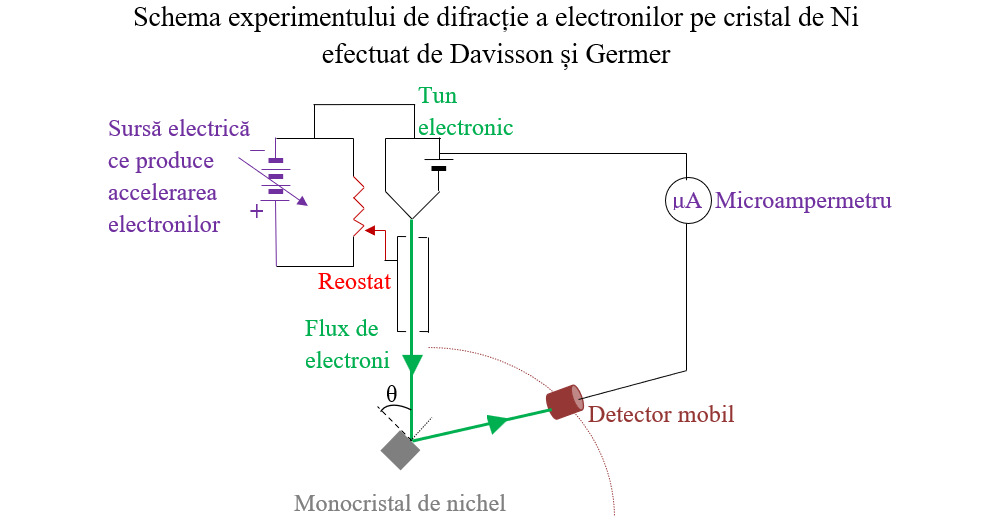
II.4. Ipoteza de Broglie. Difracția electronilor. Fizichim
Według hipotezy de Broglie'a dualizmu korpuskularno-falowego każdy obiekt materialny może być opisywany na dwa sposoby: jako zbiór cząstek albo jako fala. Obserwuje się efekty potwierdzające falową naturę materii w postaci dyfrakcji cząstek elementarnych, a nawet całych jąder atomowych .

Mechanika falowa podstawy Hipoteza de Broglie a Zarówno promieniowanie
De Broglie successfully provided the explanation to Bohr's assumption by his hypothesis. Today we know that every particle exhibits both matter and wave nature. This is called wave-particle duality. The concept that matter behaves like wave is called the de Broglie hypothesis, named after Louis de Broglie, who proposed it in 1924.

Elementy fizyki kwantowej
Hipoteza de Broglie'a Czy to nie ciekawe? Stwór na tym zdjęciu wygląda, jak groźny przybysz z kosmosu. Ale widujemy te stworzenia często, jak całymi stadami latają nad miską z owocami. To maleńka muszka owocowa, sfotografowana za pomocą mikroskopu elektronowego. W mikroskopie tym zamiast światłem, badany obiekt „oświetla" się elektronami.

Hipótesis de De Broglie Examen Fisica Selectividad YouTube
Well, let's first review how we define the phase velocity. The phase velocity is the speed at which a particular point on the wave moves through space. This particular point has a constant phase, so let's set the phase in the standard definition of a wave, Ψ(x, t) = A sin(kx − ωt) (5.6.3) (5.6.3) Ψ ( x, t) = A sin ( k x − ω t) equal.
.jpg)
Victor de Broglie Biography Topics referred to by the same term
The De Broglie hypothesis proposes that all matter exhibits wave-like properties and relates the observed wavelength of matter to its momentum. After Albert Einstein's photon theory became accepted, the question became whether this was true only for light or whether material objects also exhibited wave-like behavior.

De broglie wavelength YouTube
Hipoteza de Broglie'a Liceum ogólnokształcące i technikum Fizyka długość fali cząstki dualizm korpuskularno-falowy fale materii hipoteza de Broglie'a Udostępnij Wprowadzenie Przeczytaj Film samouczek Sprawdź się Dla nauczyciela Tekst: Krystyna Wosińska Politechnika Warszawska - Wydział Fizyki

Contoh Soal Fisika Kuantum Kelas 12
While this equation was specifically for waves, de Broglie, using his hypothesis that particles can act like waves, combined the equations: E = m c 2 = h ν. Where E is energy, m is mass, c is the.
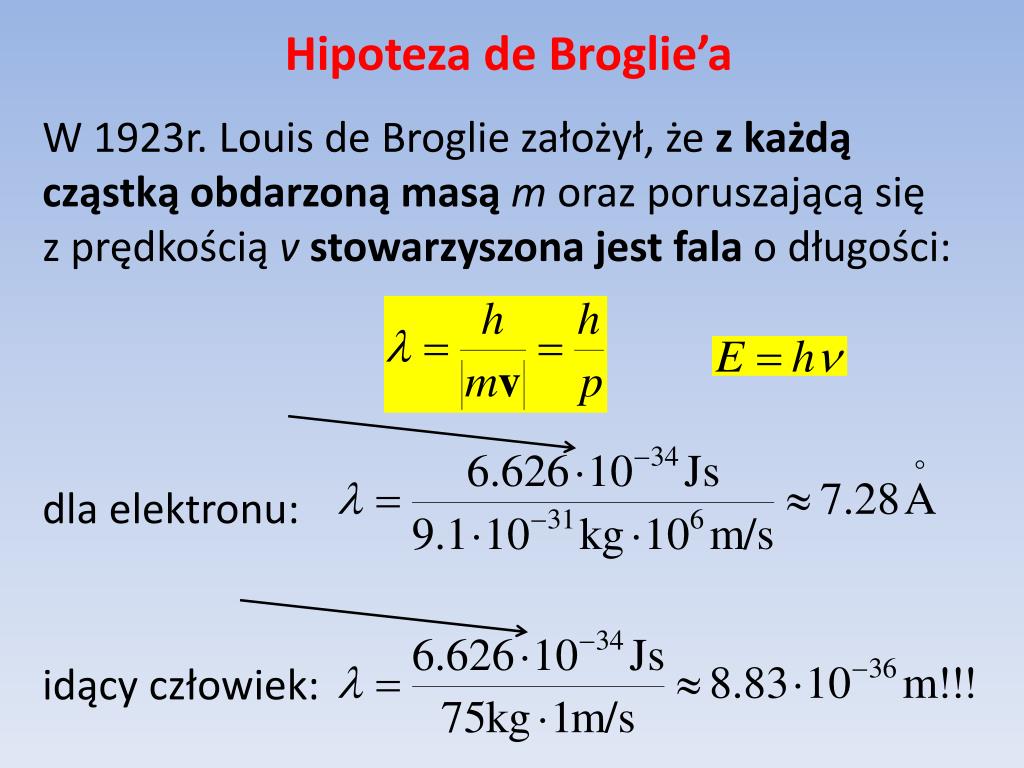
PPT Mechanika Kwantowa PowerPoint Presentation, free download ID
Hipotesis de broglie menyatakan bahwa setiap partikel yang memiliki momentum linear dapat berperilaku sebagai gelombang. Namun, sebenarnya apa itu hipotesis de Broglie? Untuk mengetahui jawabannya, simaklah penjelasan di bawah ini! Latar belakang hipotesis de Broglie
What are the important properties of the De Broglie's waves? Quora
În fizică, ipoteza De Broglie este afirmația că materia (orice obiect) are o natură ondulatorie ( dualitatea undă-corpuscul ). Relațiile De Broglie arată că lungimea de undă este invers proporțională cu impulsul unei particule și că frecvența este direct proporțională cu energia cinetică a particulei.

De Broljeva Hipoteza PDF
Louis de Broglie, (born August 15, 1892, Dieppe, France—died March 19, 1987, Louveciennes), French physicist best known for his research on quantum theory and for predicting the wave nature of electrons.He was awarded the 1929 Nobel Prize for Physics.. Early life. De Broglie was the second son of a member of the French nobility. From the Broglie family, whose name is taken from a small town.

De Broglie's wavelength YouTube
De Broglie je 1929. dobio Nobelovu nagradu za svoju teoriju (prvi put je ikada dodijeljena za doktorsku tezu), a Davisson/Germer su je zajedno osvojili 1937. za eksperimentalno otkriće difrakcije elektrona (a time i dokazivanje de Broglieove hipoteza).. Iako de Broglieova hipoteza predviđa valne dužine za bilo koju veličinu, postoje.

PODSTAWY MECHANIKI KWANTOWEJ De Broglie, na podstawie analogii
De Broglie-eva hipoteza: Biografija: Uvod: De Broglie-eva hipoteza: Galerija: Kviz . Svakoj čestici koja se kreće brzinom v i ima impuls p, može se pridružiti, talas, talasne dužine: Prethodna relacija naziva se de Broljeva relacija. Talas pridružen čestici naziva se de Broljev talas, a talasna dužina talasa koji je pridružen čestici.

velocity of deBroglie wave YouTube
Hipoteza e de Broglie tregoi se dualiteti i grimcave të valëve nuk ishte thjesht një sjellje e gabuar e dritës, por përkundrazi ishte një parim themelor i ekspozuar nga rrezatimi dhe materia.

33+ de broglie wavelength calculator KeirraLeandro
Tako je De Brolj svojom hipotezom ukazao na teorijsku osnovu drugog Borovog postulata, da su orbite elektrona kvantizovane, odnosno da stabilna energetska stanja postoje samo ako je ispunjen uslov da je moment impulsa elektrona (L) jednak celobrojnom umnošku redukovane Plankove konstante ћ (čita se "ash").